Abstract:
Different mechanical forces play an important role in all stages of human life, from the embryonic period (morphogenesis) to aging, through the development, maturation and maintenance of organs and tissues. Mechanical stimuli also play a key role in regenerative medicine, as it has been demonstrated that stem cells undergo differentiation under mechanical stimulation.
Mechanobiology is the study of how mechanical forces influence biological systems. It is a highly interdisciplinary field of research at the interface between biology, medicine, physics, biomaterial science, and bioengineering. It investigates the interactions between mechanical forces and biological processes at various scales, from molecules to tissues. The purpose of this communication is to introduce the emerging field of Mechanobiology in regenerative medicine and to highlight its growing importance in our daily work.
Stem cells can sense mechanical cues through specialized proteins and structures. This process, called mechanosensing, is responsible for how stem cells detect and respond to their mechanical environment, and how these responses influence their fate, such as proliferation, differentiation, and self-renewal.
In both the aging process and facial reshaping, understanding and controlling the mechanical stimuli and dynamics within tissues, as well as their consequences, constitute one of the fields of research and development of Morphodynamic Cosmetic Surgery.
1. WHAT IS MECHANOBIOLOGY
Mechanobiology is the interdisciplinary science that studies how physical forces and mechanical properties of the cellular microenvironment regulate biological functions at multiple scales, from molecules to organs (Ingber, 2006; Geiger et al., 2009).
2. HOW IT MATTERS
Mechanical cues are as important as biochemical factors in determining cell fate (Discher et al., 2009; Engler et al., 2006). Dysregulation of these cues contributes to cicatricial fibrosis, degenerative diseases, and accelerated aging (Humphrey et al., 2014).
3. ROLE OF MECHABIOLOGY IN REGENERATIVE AESTHETIC MEDICINE AND MDCS
Recent advances in stem cell biology demonstrate that mechanical stimuli regulate proliferation, differentiation, and self-renewal through a process known as mechanosensing [Argentati, 2019, Vining, 2017]. Specialized proteins and cytoskeletal structures translate physical cues into biochemical signals, guiding stem cell commitment toward osteogenic, chondrogenic, or adipogenic lineages [Wang, 2023, Martino, 2018]. This finding is crucial in regenerative aesthetic medicine, where subtle and controlled mechanical forces can be harnessed to induce neocollagenesis, elastogenesis, adipose tissue and bone remodeling.
Mechanobiology provides a framework for using controlled physical stimulation to activate stem cells and guide tissue repair (Vining & Mooney, 2017; Robling et al., 2006; Murphy et al., 2014).
4. MECHANOBIOLOGY AND EXOSOMES
Mechanical forces regulate paracrine signaling through exosomes. Exosome release increases under shear stress and cyclic strain, carrying growth factors and RNAs that enhance regeneration (Trubiani et al., 2019; Chen et al., 2024).
5. ROLE OF MECHANOBIOLOGY IN MORPHODYNAMIC COSMETIC SURGERY
Mechanobiology has a fundamental role in MDCS.
All the principles of MDCS are based on Mechanobiology.
MDCS applies mechanobiology to analyze vectors of muscular activity, tissue tension, and bone remodeling. In this way it is possible to obtain an effective rejuvenation of the tissues, to permanently modify the facial dynamics even in young subjects, this avoiding having to undergo more invasive interventions. Treatments reprogram mechanical dynamics to restore harmony (Rizzo, 2020).
6. FUNCTIONAL ANATOMY AND MECHANOBIOLOGY
Functional anatomy describes tissues by their dynamic interactions under load. Chronic micro-tensions influence collagen turnover, fat distribution, and skeletal adaptation (Mendelson & Wong, 2012; Shaw & Kahn, 2007).
7. BONE REMODELING (Muscular Activity, Hyperactivity, Hypoactivity, Botox, Threads)
Bone is a mechanosensitive organ. Muscular hyperactivity increases loading and reshapes bones, while hypoactivity accelerates resorption. Botox reduces strain; threads redistribute forces and stimulate fibroblasts (Bonewald, 2011; Parsons & Hallgrímsson, 2019; Huja et al., 2006; Rafferty et al., 2012; Woodbury et al., 2023, Rizzo, 202).
8. MYOMODULATION
Myomodulation refers to altering muscle dynamics with fillers, botulinum toxin, threads or devices, providing mechanical opposition or functional support (Rizzo, 2025, de Maio, 2018; Coimbra & Stefanello, 2023).
9. BIOMATERIALS AND MECHANOBIOLOGY
Biomaterials act as mechanically active scaffolds and stimuli. Hydrogels, nanocomposites, and bioresorbable threads sustain tissues and stimulate regenerative cascades (Mao & Mooney, 2015; Shi & Lv, 2024).
10. WHAT HAPPENS MODIFING NEGATIVE DYNAMICS
Negative dynamics can accelerate aging signs and can reduce the pleasantness of a face. By altering vectors (myomodulation, filler, threads, botox), destructive forces can be transformed into regenerative stimuli (Ciarletta, 2012, Rizzo, 2024).
11. AGING IS ATROPHY
Youth is mechanostimulation, aging is marked by atrophy from loss of mechanical stimuli. Youthful tissues experience constant mechanostimulation that preserves stem cell activity (Buvinic et al., 2021; Vining et al., 2019).
12. EXAMPLES
Mechanobiology is increasingly applied across medicine (Martino et al., 2018).
Linear scars act as stress lines with positive/negative effects (Gurtner et al., 2008).
Prostheses may abolish physiological signaling, leading to atrophy (van Oers & Merks, 2022).
13. MUSCLES, EXPRESSIONS, EMOTIONS, HABITS: MORPHODYNAMIC CRANIOFACIAL ANALYSIS (MD-CFA)
13.1 Muscular Architecture and Force Vectors
The face is a tensegrity system in which mimetic muscles, SMAS, retaining ligaments and fascia distribute loads to skin and bone. Each muscle has a dominant vector that affects regional morphology (Parsons & Hallgrímsson, 2019; Mendelson & Wong, 2012). Chronic overpull of depressors (DAO, depressor septi, platysma) engraves folds and drives caudal drift; underactivity of elevators reduces cranial support, favoring pseudo‑ptosis (Shaw & Kahn, 2007).
MD‑CFA maps these vectors at rest and during key expressions to identify targets for myomodulation (Rizzo, 2024, de Maio, 2018).
13.2 EXPRESSION AS REPEATED LOADING PATTERN
Dynamic expressions act as cyclical mechanical loading. Micro‑creases become macro‑wrinkles when cycles and amplitude exceed dermal repair capacity, shifting fibroblasts toward a pro‑fibrotic state (Gurtner et al., 2008). Conversely, balanced cyclical loading sustains collagen turnover and elastic fiber alignment.
MD‑CFA quantifies ‘expression dose’ (frequency × amplitude × duration) for smile, brow raise, frown, squint and pursing (Rizzo, 2014, Vining & Mooney, 2017).
13.3 EMOTIONS-DRIVEN MOTOR PROGRAMS
Emotions that persist for a long time, such as sadness, anxiety, depressions, distrust, etc, can permanently modify some facial dynamics and lead to evident changes in the face (Rizzo, 2020).
Emotion programs recruit stereotyped motor synergies that repeatedly load the same tissues. Over years, these loads influence soft‑tissue thickness, crease depth and even bony appositions at entheses through osteocyte mechanosensing (Bonewald, 2011).
MD‑CFA tracks the patient’s predominant emotional repertoire and correlates it with morphologic signatures (Rizzo, 2024).
13.4 HABITS AND LIFESTYLE AS LONG-TERM FORCE MODULATORS
Functional habits calibrate the baseline mechanical milieu: mastication side‑dominance, parafunctions (bruxism, clenching), breathing route, posture (‘tech‑neck’), speech, sleep position, etc. Unilateral chewing and bruxism increase masseter load; mouth breathing alters tongue posture, chin evolution and the trophysm of maxillary bones and zygomatic area (Rizzo, 2020); head‑forward posture increases platysma traction (Robling et al., 2006; Parsons & Hallgrímsson, 2019).
13.5 NEGATIVE VS POSITIVE DYNAMICS MAPPING
MD‑CFA classifies vectors as negative (aging‑accelerating or pejorative) or positive (youth‑preserving or beautification).
Some of negative dynamics include caudal vectors from depressors, chronic perioral compression, periorbital over‑squint and asymmetric mastication; some of positive dynamics include balanced zygomatic elevation, efficient nasal breathing and adequate masticatory loading (Humphrey et al., 2014; Rizzo, 2024).
13.6 MD‑CFA CAPTURE AND MEASUREMENT PROTOCOL
Acquisition combines static and dynamic datasets: images and photos at rest and during expressions; video loops for motion analysis; the Morphodynamic Cranio-Facial Analysis is performed on these images. Outputs include Facial Landmarks, Vector Maps, Strain Indices, Symmetry Scores, Tissue Stiffness Scores, Muscle Mapping, etc, (Rizzo, 2024).
13.7 DECISION MATRIX AND TREATMENT ALGORITHMS
MD‑CFA converts measurements into an intervention map: Myomodulation (fillers, spacers, neuromodulators) (de Maio, 2018; Harris, 2019); Thread‑based vector re‑education (Lim et al., 2022; Kim et al., 2021); Biomaterial selection by mechanotype (Engler et al., 2006; Murphy et al., 2014); Habit reprogramming (Robling et al., 2006); Bone‑sparing strategy (Hong & Kang, 2020; Xie et al., 2014), Morphodynamica Cosmetici Surgery techniques (Rizzo, 2024).
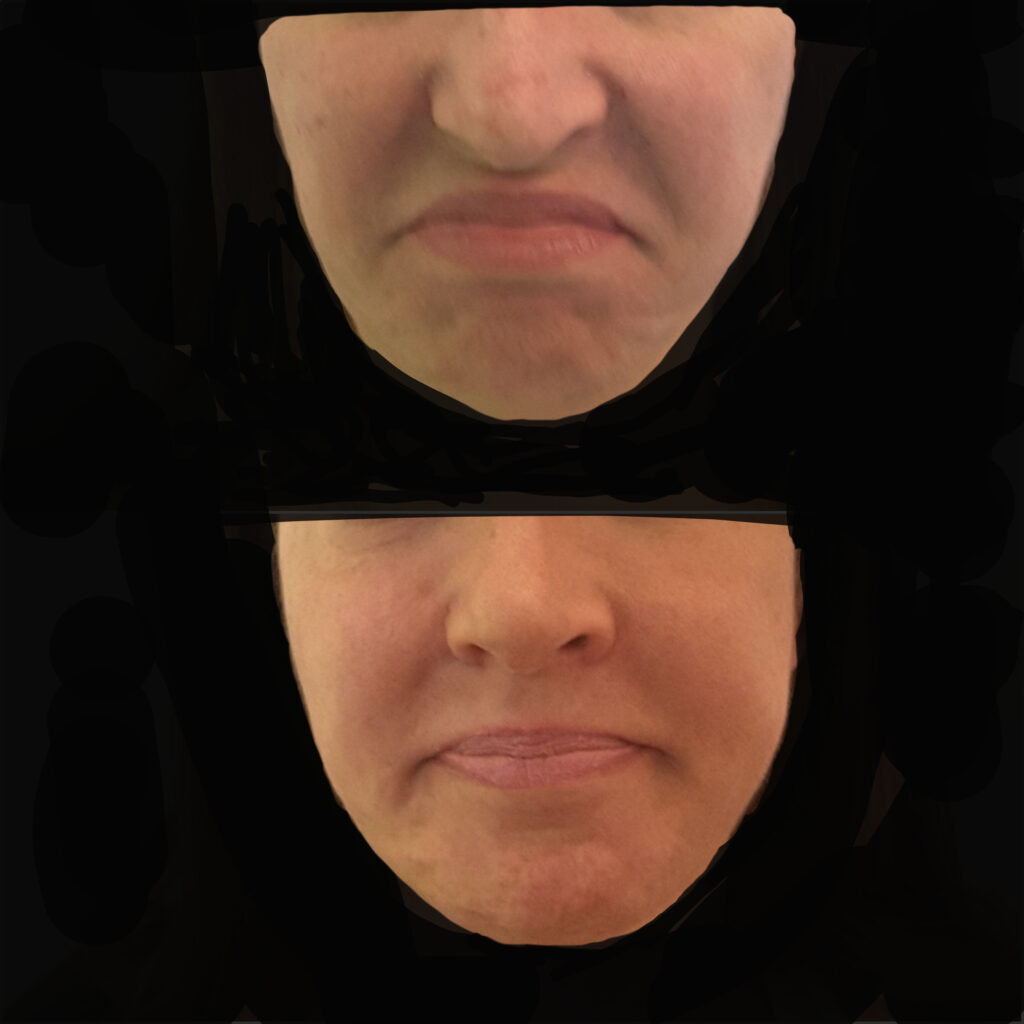
14. Cases
Case A:
In this patient we find: hypoactivity of the lateral bundles of the frontalis muscle with sagging of the upper eyelid, hyperactivity of the corrugators, of the depressors muscles of the lower third of the face, oral breathing, evident tear throughs and nasolabial folds.
Treatments plan: Thread reshaping of the frontal, temporal, zygomatic, and malar regions, reduction of the membranous septum of the columella, and resection of the depressor nasal tip muscle. Treatment of the lower third with botulinum toxin.

Case B:
The objectives of the treatments received by the second patient were beautification and rejuvenation, using threads the lateral frontal, temporal, zygomatic and malar areas were expanded, the projection of the chin was increased, ultimately the V shape of the face was accentuated.

Case C:
In the third patient, the dynamics of the nasal muscles and depressor muscles were modified.

References
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20(7):811–827.
2. Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10(1):21–33.
3. Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324(5935):1673–1677
4. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689.
5. Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–812.
6. Argentati C. et al. Insight into Mechanobiology: How Stem Cells Feel Mechanical Forces and Orchestrate Biological Functions. Int J Mol Sci. 2019;20(21):5337.
7. Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017;18(12):728–742.
8. Wang L. et al. Cell response to mechanical microenvironment cues via Rho signaling: From mechanobiology to mechanomedicine. Acta Biomater. 2023;155:68–82.
9. Martino F, et al. Cellular Mechanotransduction: From Tension to Function. Front Physiol. 2018;9:824.
10. Vining KH, Stafford A, Mooney DJ. Regulating stem cell fate with mechanical cues. Annu Rev Biomed Eng. 2019;21:431–456.26.
11. Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–498.
12. Murphy WL, McDevitt TC, Engler AJ. Materials as stem cell regulators. Nat Mater. 2014;13(6):547–557.
13. Trubiani O, Marconi GD, et al. Mechanobiology of human periodontal ligament stem cells: insights into exosome biogenesis and function. Front Physiol. 2019;10:1509.
14. Chen Y, et al. Dynamic nanomechanical characterization of cells in exosome therapy. Microsyst Nanoeng. 2024;10:97.
15. Rizzo, A. “Morphodynamic Cosmetic Surgery: holistic beauty – The new paradigms of aesthetic surgery”. Amazon Direct Publishing. 2020
16. Mendelson B, Wong CH. Changes in the facial skeleton with aging. Aesthetic Plast Surg. 2012;36(4):753–760.
17. Shaw RB, Kahn DM. Aging of the facial skeleton. Plast Reconstr Surg. 2007;119(2):675–682.
18. Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–238.
19. Parsons TE, Hallgrímsson B. Muscle-induced loading as an important source of variation in craniofacial skeletal shape. Genesis. 2019;57(1):e23263.
20. Huja SS, Fernandez SA, Hill KJ, Li Y. Remodeling dynamics in the alveolar process in skeletally mature dogs. Anat Rec A Discov Mol Cell Evol Biol. 2006;288(12):1243–1249.
21. Rafferty KL, Liu ZJ, Ye W, et al. Botulinum toxin in masticatory muscles: effects on muscle, bone, and craniofacial function. Bone. 2012;50(3):651–662.
22. Woodbury SM, et al. Mechanobiology-informed biomaterial and tissue engineering strategies. Front Physiol. 2023;14:1220555.
23. Rizzo, A., 2025. Morphodynamic craniofacial analysis: a new perspective in aesthetic medicine. Chirurgia Cosmetica Morfodinamica, [online] 21 October. Available at: https://chirurgiacosmeticamorfodinamica.it/morphodynamic-craniofacial-analysis
24. de Maio M. Myomodulation with Injectable Fillers. Aesthetic Plast Surg. 2018;42(3):798–814.
25. Coimbra DDA, Stefanello B. Myomodulation with facial fillers. Aesthetic Plast Surg. 2023;47(3):1162–1174.
26. Mao AS, Mooney DJ. Regenerative medicine: Current therapies and future directions. Proc Natl Acad Sci USA. 2015;112(47):14452–14459.
27. Shi K, Lv X. Mechanically active supramolecular systems. Small Sci. 2024;4(1):2300300.
28. Ciarletta P. On the biomechanics and mechanobiology of growing skin. J Theor Biol. 2012;297:166–175.24.
29. Buvinic S, et al. Muscle-bone crosstalk in the masticatory system. Front Endocrinol. 2021;11:606947.
30. Vining KH, Stafford A, Mooney DJ. Regulating stem cell fate with mechanical cues. Annu Rev Biomed Eng. 2019;21:431–456.
31. Rizzo A. Morphodynamic cosmetic surgery: functional facial dynamics and regenerative perspectives [Internet]. Chirurgia Cosmetica Morfodinamica; 2024. Available from: https://chirurgiacosmeticamorfodinamica.it
32. Gurtner GC, Wong VW, Longaker MT. Scar formation and wound healing. J Clin Invest. 2008;118(3):789–801.
33. van Oers RF, Merks RM. Multiscale mechanobiology. J R Soc Interface. 2022;19(189):20210856.
34. Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20(7):811–827.
35. Rizzo A. “La Chirurgia Cosmetica Morfodinamica: la bellezza olistica – I nuovi paradigmi della Chirurgia estetica”. Amazon Direct Publishing.2020.
36. Rizzo, A. “La Rinoplastica Morfodinamica. Alla ricerca dell’armonia: connubio tra estetica, funzione e benessere”. Amazon Direct Publishing. 2020.
37. Rizzo, A. “Morphodynamic Cosmetic Surgery: holistic beauty – The new paradigms of aesthetic surgery”. Amazon Direct Publishing. 2020
38. Naqvi S.N. Stem Cell Mechanobiology and the Role of Biomaterials in Governing Mechanotransduction and Matrix Production for Tissue Regeneration. Front Bioen Biotechnol. 2020;8:597661.
39. Rizzo, A., 2025. Morphodynamic craniofacial analysis: a new perspective in aesthetic medicine. Chirurgia Cosmetica Morfodinamica, [online] 21 October. Available at: https://chirurgiacosmeticamorfodinamica.it/morphodynamic-craniofacial-analysis
40. Rizzo, A. 2025. From facial dynamics to Regenerative Aesthetic Medicine. Chirurgia Cosmetica Morfodinamica. [on line] August. Available at: https://www.chirurgiacosmeticamorfodinamica.it/2025/08/26/morphodynamic-cosmetic-surgey-from-facial-dynamics-to-regenerative-aesthetic-medicine-a-new-paradigm-in-aesthetic-surgery/
41. Rizzo, A. 2024. Meccanobiologia. Chirurgia Cosmetica Morfodinamica. [on line] May. Available at: https://www.chirurgiacosmeticamorfodinamica.it/2024/05/20/meccanobiologia/



